Pipeline
About torudokimab
torudokimab
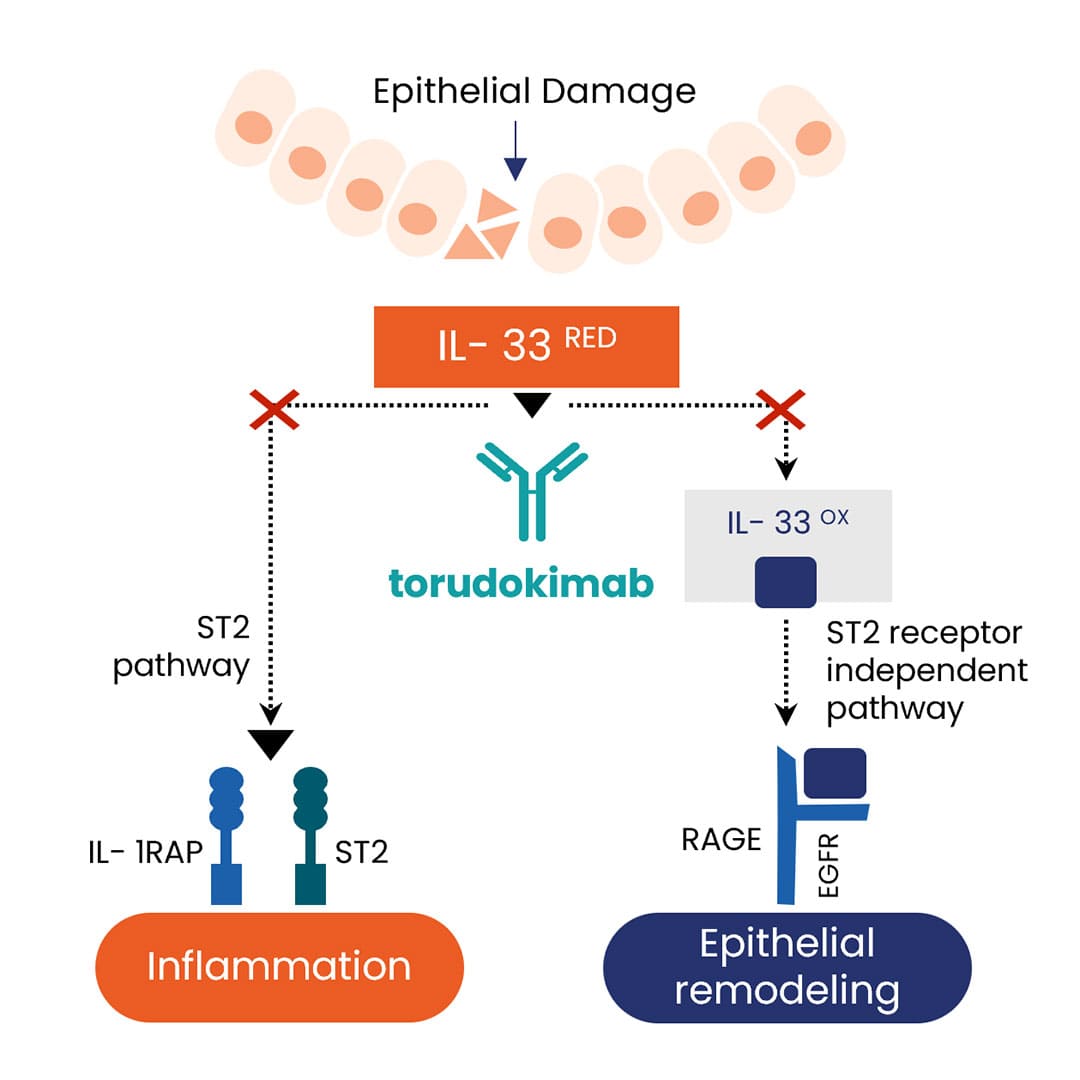
Torudokimab (ZB-880) is a fully human, high affinity monoclonal antibody that neutralizes IL-33, preventing ST2-dependent and ST2-independent (e.g., RAGE) inflammation.
Torudokimab has been well tolerated to date. In 244 subjects dosed with torudokimab to date, the majority of adverse events were categorized as grade 1 or grade 2. There were no deaths to date, or any serious AEs causally related to torudokimab.
Torudokimab can be administered by subcutaneous (“SC”) injection or intravenous (“IV”) infusion. In clinical studies, torudokimab has been dosed in healthy volunteers using an SC and IV formulation and in patients using a SC formulation.
We plan to conduct necessary CMC and regulatory activities to prepare torudokimab for Phase 2 readiness in allergy or respiratory related indications. Additionally, we are monitoring Phase 2 and Phase 3 external catalysts, particularly in asthma and chronic obstructive pulmonary disease.
IL-33 Pathway
Targeting IL-33 In Epithelial Driven Diseases
IL-33 is a member of the IL-1 cytokine family that is constitutively expressed in structural and lining cells including fibroblasts, endothelial, and epithelial cells of skin, gastrointestinal tract, and lungs
IL-33 is released from the epithelium by disease exacerbating environmental factors and is an important amplifier of innate inflammation during exacerbations
Polymorphisms in IL-33 and ST2 are associated with increased eosinophil production as well as respiratory diseases such as COPD and Severe Asthma.
References
- IL-33: Roles in Allergic Inflammation and Therapeutic Perspectives
Chan BCL, Lam CWK, Tam L-S and Wong CK (2019) Front. Immunol. 10:364. doi: 10.3389/fimmu.2019.00364 - Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation
Cohen, E., Scott, I., Majithiya, J. et al. Nat Commun 6, 8327 (2015). https://doi.org/10.1038/ncomms9327 - Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma
Wechsler ME, Ruddy MK, Pavord ID, Israel E, Rabe KF, Ford LB, Maspero JF, Abdulai RM, Hu CC, Martincova R, Jessel A, Nivens MC, Amin N, Weinreich DM, Yancopoulos GD, Goulaouic H. N Engl J Med. 2021 Oct 28;385(18):1656-1668. doi: 10.1056/NEJMoa2024257
