Pipeline
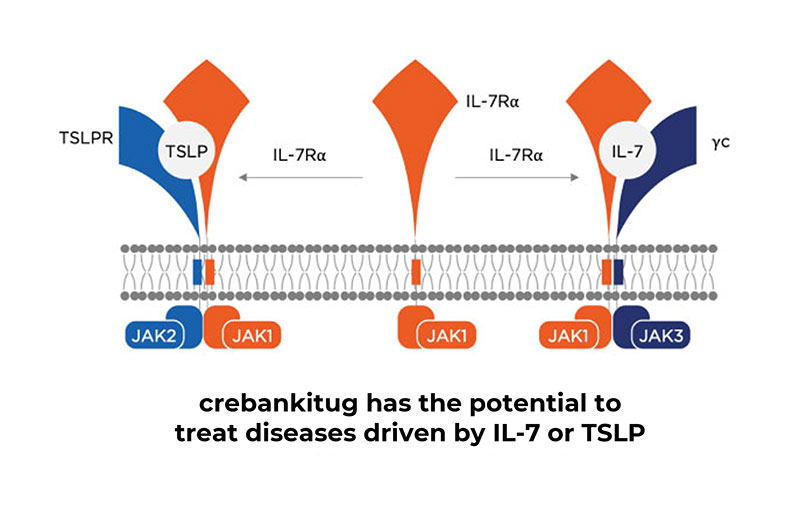
crebankitug
Crebankitug has been well tolerated to date. In 93 subjects dosed with crebankitug to date, the majority of adverse events (“AEs”) were categorized as grade 1 or grade 2. There were no deaths or permanent discontinuations due to AEs.
Zura Bio holds an exclusive worldwide license for the development and commercialization of crebankitug in all indications.
It is the only anti-IL-7Rα program to date with clinical data showing impact on key T-cell subpopulations, underscoring its potential applicability in a broad range of T-cell mediated diseases and atopic diseases.
We plan to conduct necessary CMC and regulatory activities to prepare crebankitug for Phase 2 readiness. Additionally, we are actively monitoring Phase 2 IL-7R external catalysts along with additional TSLP-driven catalysts.
IL-7 and TLSP Pathways
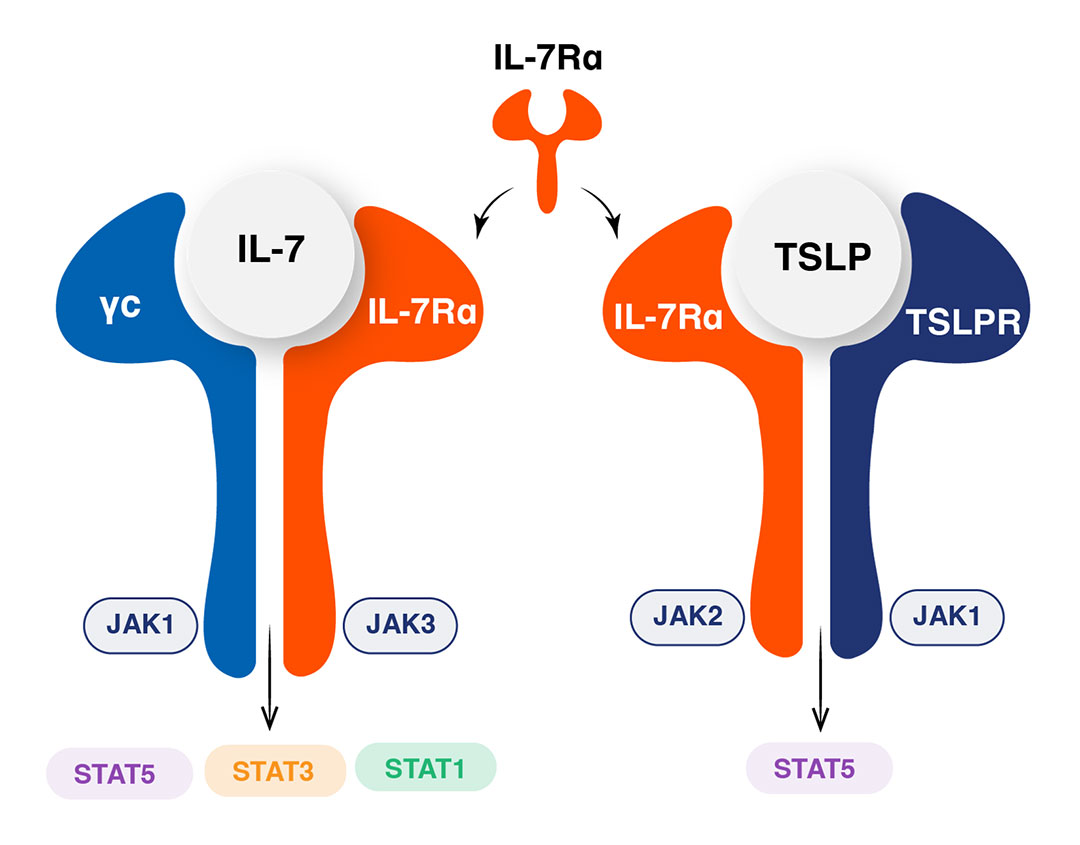
IL-7Rα: A unique target for modulating two key immune pathways
IL-7 Pathway
IL-7 is a critical modulator of T-cell homeostasis,
proliferation, and survival
Inhibition of IL-7Rα promotes a normalisation in Treg :Teffector T-cell ratios
Polymorphism in IL-7Rα associated with multiple disease states, including T1DM, MS, UC, Sarcoidosis PBC and AA
TSLP Pathway
Is a regulator of Th2 immune response
Is involved in immunity at barrier surfaces
(skin, airways, gut)
Is clinically validated for the treatment of
severe asthma
Is under clinical investigation in CSU, COPD,
and chronic rhinosinusitis
References
- Immunomodulatory activity of humanized anti–IL-7R monoclonal antibody RN168 in subjects with type 1 diabetes
Herold et al, JCI Insight. 2019;4(24):e126054. https://doi.org/10.1172/jci.insight.126054 - Model-Based Characterization of the Pharmacokinetics, Target Engagement Biomarkers, and Immunomodulatory Activity of PF-06342674, a Humanized mAb Against IL-7 Receptor-α, in Adults with Type 1 Diabetes
Williams, J.H., Udata, C., Ganguly, B.J. et al. AAPS J 22, 23 (2020). https://doi.org/10.1208/s12248-019-0401-3 - Blockade of IL-7 signaling suppresses inflammatory responses and reverses alopecia areata in C3H/HeJ mice
Dai, Zhenpeng et al. Science advances vol. 7,14 eabd1866. 2 Apr. 2021. doi: 10.1126/sciadv.abd1866.
