Pipeline
About tibulizumab
tibulizumab
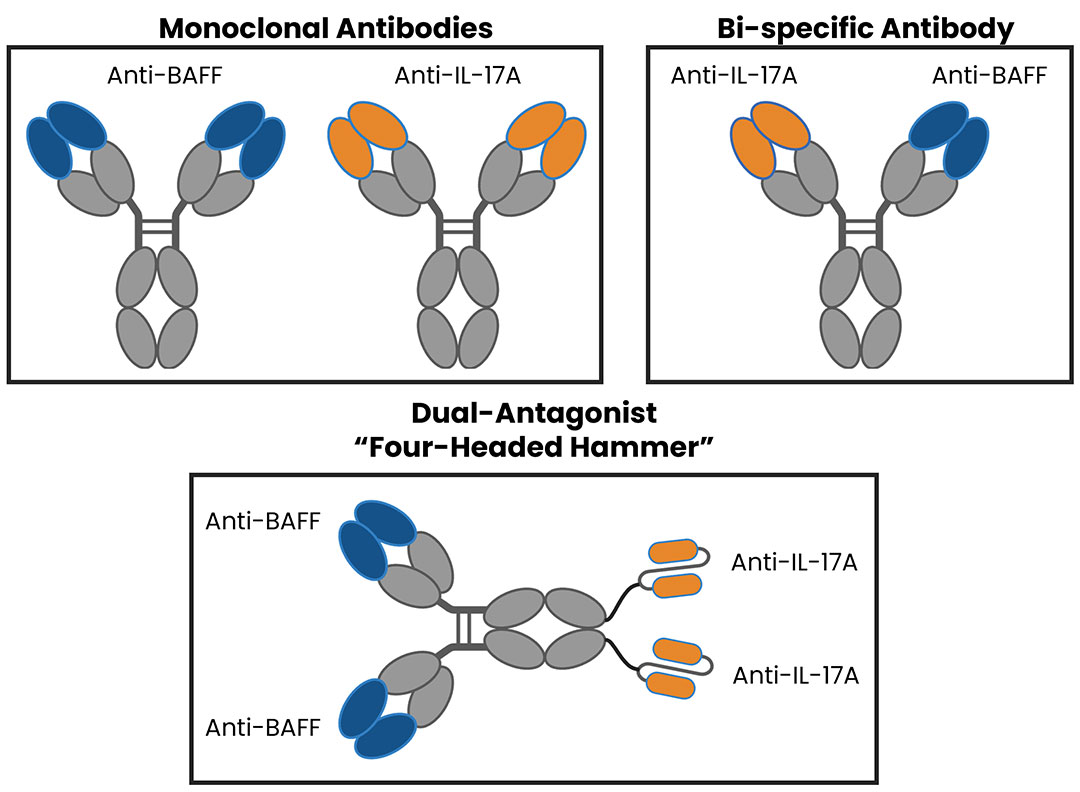
Tibulizumab (ZB-106) is a potential first-in-class, anti-IL-17 and anti-BAFF dual antagonist engineered by the fusion of Taltz® (ixekizumab) and tabalumab.
Tibulizumab does not require dual target engagement to achieve biological activity, it is a single agent combination of two independently acting bivalent antagonist moieties. In this respect, it can be thought of as a “four-headed hammer” that can simultaneously knock out two distinct soluble ligands.
To date, 78 study participants have been dosed with tibulizumab across three Phase 1b studies, including two completed studies in rheumatoid arthritis and Sjögren’s syndrome. The safety profile to date appears to be acceptable, with no new findings relative to known IL-17 and BAFF inhibitors. Chronic toxicology studies have been completed with no adverse drug-related findings.
The half-life of tibulizumab is 26.9 days.
Two Phase 2 trials are currently recruiting to evaluate tibulizumab in adults with autoimmune conditions: TibuSURE in systemic sclerosis and TibuSHIELD in hidradenitis suppurativa.
Anti IL-17A / BAFF (BAFF + 17)
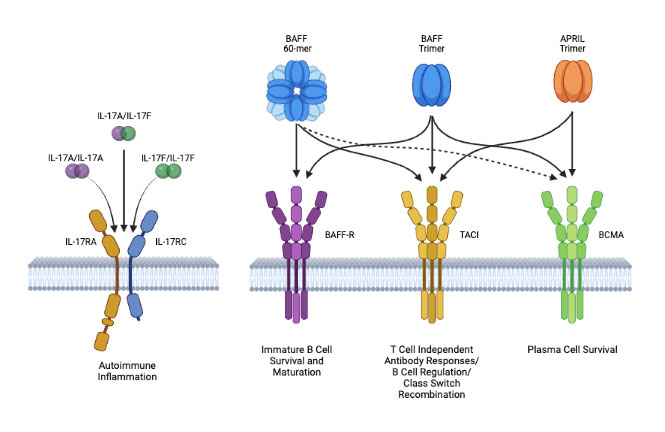
Tibulizumab disrupts IL-17A and/or BAFF-Mediated Inflammation
Tibulizumab has the potential to treat diseases driven by B-cell and T-cell signalings
T-cell and B-cell synergy
Multiple T-cell driven diseases remain sub-optimally treated despite the growth in “pure play” anti-IL-17A drugs
B-cell driven diseases often have a T-cell driven autoimmune component contributing to the pathology
Combined approach to address T-cell and B-cell drivers of autoimmunity has the potential to increase clinical benefit
HIDRADENITIS SUPPURATIVA
Hidradenitis suppurativa is an inflammatory follicular skin disease with US estimates of 300,000 to 400,000 HS patients.
Significant opportunity and clinical need in hidradenitis suppurativa exist. The combined IL-17 + BAFF inhibition potentially results in broader and improved clinical responses for HS patients. Tibulizumab may also offer convenient Q4W SC dosing regimen.
SYSTEMIC SCLEROSIS
Systemic sclerosis is a rare and potentially fatal autoimmune disease characterized by tissue inflammation and fibrosis. There are approximately 300,000 individuals affected in the U.S., E.U., and Japan.
There is a high unmet medical need with insufficient therapy currently available for patients living with this rheumatic disease. Currently, two disease-modifying drugs are approved for severe lung complications of the disease (SSc-ILD), but no effective treatment exists that combats the disease across organ systems.
Tibulizumab’s unique dual-pathway biology targets both the IL-17 and BAFF pathways, represents a potential first-in-class therapy that may potentially offer a paradigm shift in care, if approved. Tibulizumab’s convenient Q4W subcutaneous dosing schedule adds to its appeal as a potential treatment option.
DOWNLOAD THE SYSTEMIC SCLEROSIS (SSc) DISEASE STATE FACT SHEET
References
- Liu et al. 2016. J Inflam Research
- Manetta et al. 2014. J Inflam Research
- Benschop et al. 2019. Mabs
- Smulski and Eibel. 2018. FrontImmunol
